After 2 reported deaths, officials tell Ixchiq users age 60+ to stop vaccine temporarily
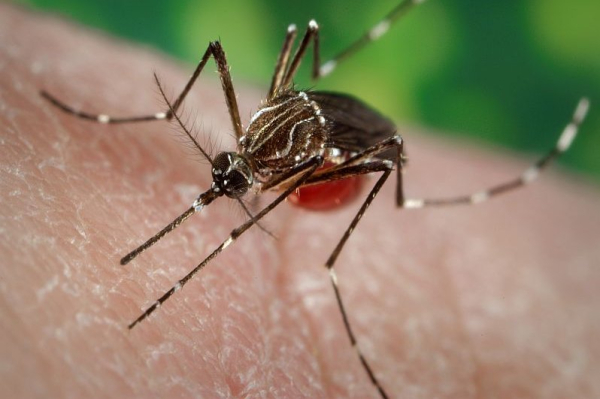
In November 2023, the FDA approved Ixchiq for the prevention of disease caused by the chikungunya virus in patients age 18 and older. Chikungunya is an infection caused by the chikungunya virus and is spread by mosquitoes. File Photo courtesy the Centers for Disease Control and Prevention
U.S. health officials are advising patients using the drug Ixchiq to hold off while authorities seek newer information on vaccine results in the global fight against chikungunya.
The pause for U.S. patients was initiated Friday by the FDA and CDC while the two federal agencies investigate “postmarketing reports of serious adverse events, including neurologic and cardiac events, in individuals who have received the vaccine” Ixchiq, otherwise known as live “Chikungunya Vaccine,” according to a safety communication by the U.S. Food and Drug Administration.
In November 2023, the FDA approved Ixchiq for the prevention of disease caused by the chikungunya virus in patients age 18 years and older.
On Friday, health officials said the FDA will conduct an updated benefit-risk assessment for the use of Ixchiq in individuals 60 years of age and older after over 80,000 doses of Ixchiq were distributed worldwide.
Since the start of this year, roughly 80,000 cases and 46 CHIKVD-related deaths have been reported in 14 countries or territories, says the EU-backed European Centre for Disease Prevention and Control.
FDA officials said as of Wednesday that 17 “serious adverse events” were reported globally in people over age 62 who got Ixchiq, with two reported deaths. Of the 17 reports, six were U.S.-based, but American health officials did not indicate if any of those deaths were in the United States.
However, officials pointed out that reported adverse events “may not be causally related to vaccination.”
According to the U.S. Centers for Disease Control and Prevention, the virus is transmitted by mosquitoes and the symptoms can last anywhere from one week to several months.
Like dengue fever, chikungunya virus causes high fever, rashes, headache and joint pain. And while most patients recover with days, the joint pain can persist for months that can be managed with pain-relieving and anti-inflammatory medications.
But while it has the potential to temporarily disable a victim, it does not often result in death.
The single-dose shot approved November 2023 by the federal government was for use in adults with an increased risk of exposure to the chikungunya virus.
Mosquitoes can infect people with Zika and chikungunya viruses at the same time, research has suggested, and may cause fatal brain infection which hit 24 victims by November 2015.
The virus was observed and described in U.S. patients who traveled from the Caribbean area to Georgia and North Carolina as chikungunya hit Cuba around May 2014.
Chikungunya, an illness caused by a virus spread via mosquito bites but not by person-to-person contact, was first detected in the Americas near the end of 2013 as it swiftly spread to other Caribbean nations, Africa and Asia.
However, no cases have been reported on Europe’s mainland.
Friday’s federal advisory on Ixchiq arrived amid a crackdown on vaccine use and its pertinent studies lead by U.S. Health and Human Services Secretary Robert F. Kennedy Jr., a known critic of effective and long-existing vaccines.
Visit the U.S. Centers for Disease Control and Prevention for more on chikungunya virus.

