Scientists use nanoparticles to clear Alzheimer’s brain plaque in mice
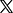
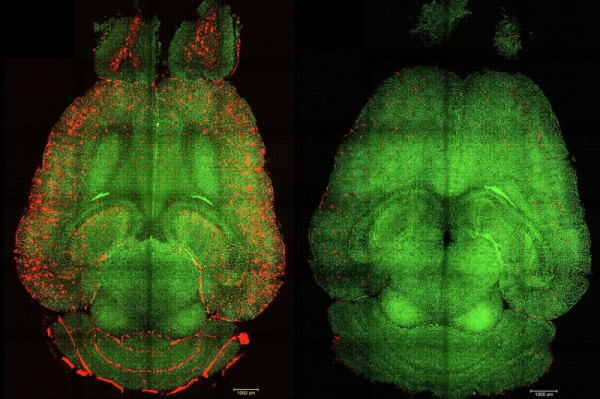
1 of 2 | Before-and-after fluorescence microscope images of a mouse brain show red-colored build-ups of toxic amyloid beta plaque (L) and the same brain 12 hours after being treated with nanoparticles. Spanish and Chinese scientists say their nanoparticle experiments could create a new focus on the blood-brain barrier in treating Alzheimer’s disease. Image courtesy Institute for Bioengineering of Catalonia
Spanish and Chinese scientists said Monday they have used specifically tailored nanoparticles to clear out build-ups of a key toxin linked to Alzheimer’s disease in the brains of mice.
Researchers from the Institute for Bioengineering of Catalonia in Barcelona, Spain, and the West China Hospital at Sichuan University announced in a newly published study they were able to “reverse” build-ups of the waste protein amyloid beta in mouse brains after only three injections with their nanoparticles.
Amyloid beta is a gummy, self-aggregating protein fragment that is the main component of toxic plaque build-up found in the brains of Alzheimer’s disease patients. It can begin to accumulate decades before a person starts exhibiting Alzheimer’s symptoms such as loss of memory and clarity of thought.
By tapping the nanoparticles, the researchers were able to repair a dysfunction in the “blood brain barrier,” which protects the brain from toxins in the body’s blood system, to allow the plaque to be transported across it and out of the brain for disposal, according to the study study published in the medical journal Signal Transduction and Targeted Therapy.
Only one hour after a series of nanoparticle injections, “we observed a reduction of 50%-60% in Amyloid beta amount inside the brain,” the authors stated, adding that the subject mouse — which was the equivalent of 90 years old in human terms and had been genetically programmed for high levels of amyloid beta — exhibited normal behavior six months after treatment, including a reversal of cognitive and memory decline.
The results show a “striking reversal of Alzheimer’s pathology” and opens a new research path by concentrating on restoring normalcy to the brain’s incredibly complex vasculature via repair of the blood-brain barrier, said lead author Giuseppe Battaglia, a research professor at the Catalonia institute and principal investigator of its Molecular Bionics Group.
“Many treatments have been designed to clear amyloid beta, the sticky protein that builds up in Alzheimer’s brains,” he told UPI in emailed comments. “Some of these can remove the plaques, but that alone hasn’t stopped memory loss or slowed the disease enough.
“Our work suggests a different approach: instead of focusing solely on removing what’s already gone wrong inside the brain, we aim to repair the system that keeps the brain healthy in the first place — its blood vessels and protective barrier.
“Restoring this barrier improves blood flow, reduces inflammation, and helps the brain recover its balance,” he said. “This approach could slow Alzheimer’s progression more effectively by treating one of its earliest and most overlooked causes, the breakdown of the brain’s own defense system.”
Battaglia said the nanoparticles work as “supramolecular drug” with the ability to “switch on” a workhorse protein within the blood-brain barrier that usually acts as a natural clearance system for toxic species like amyloid beta but which breaks down in Alzheimer’s patients.
“The blood-brain barrier, or BBB, is the brain’s security system: a vast network of tiny blood vessels that protect and nourish every neuron,” he explained. “For every brain cell, there is approximately one capillary that brings oxygen and removes waste. When this system falters, the brain’s delicate balance is disrupted.”
In Alzheimer’s disease, the BBB starts to break down early on, and it is indeed one of the earliest warning signs of disease onset: More than 90% of patients show signs of vascular damage even before memory problems appear. The barrier becomes “leaky” and allows harmful molecules to enter the brain, making it harder to clear waste and accelerating the disease.
“Our research focuses on helping the brain repair its own blood vessels and restore the barrier’s natural function,” the researcher said. “By supporting this recovery process, we’ve found that brain health improves and therapies become much more effective, since drugs can reach their target areas and the brain can better protect itself.”
The need for new treatment methods for Alzheimer’s remains acute. Nearly one in nine Americans over age 65 have Alzheimer’s, while for those 85 or older, the chances are one in five.
Focusing on BBB dysfunction in Alzheimer’s is widely believed to have “great significance” for both the early detection of the disease and the in-depth study of its workings. The barrier is essential to filter out larger molecules that might otherwise enter the brain through the bloodstream, thus keeping bacteria, viruses and toxic substances from invading the brain.
It also serves to transport toxins such as amyloid beta from inside the brain across its tightly packed cells for disposal in the bloodstream — a function that remains poorly understood. When healthy, the BBB taps a signaling receptor protein called LRP1 to do the job, but in Alzheimer’s patients, LRP1 signaling breaks down.
The nanoparticles used in the current study appear to jump-start the LRP1 clearance process by mimicking the protein in binding to the plaque, helping to “restore the vasculature’s natural role as a waste-clearing pathway and bring it back to proper function.”
Learning more about the role of the BBB is “an important and exciting area of research” that has attracted support from the Alzheimer’s Association although it remains in its infancy, a leader of the nonprofit said in comments issued to UPI.
Courtney Kloske, the association’s director of scientific engagement, said her group has supported several studies on the BBB, including studies that explore how damage to the BBB may impact progression of the disease, as well as work to increase the amount of drugs that can get across the barrier.
“There are many ongoing efforts in animal models to find innovative methods of crossing the BBB to improve efficiency and precision for drug delivery, but we are still a long way from these technologies being proven safe and effective in humans,” she cautioned.
The Spanish-Chinese study is based on research in a mouse model of Alzheimer’s, and while animal models of the disease are “somewhat similar to how Alzheimer’s progresses in humans, they do not replicate the disease in humans exactly,” she noted. “Models are important in helping us understand the basic biology of the disease, but we need human studies in representative populations for ideas to be fully validated.
“While these are intriguing findings, more research is needed to understand the underlying mechanisms of the BBB, and the potential impacts and outcomes of these compounds on people living with, or at risk for, Alzheimer’s,” Kloske said.

